Introduction to Medical Device Risk Management
Medical device risk management, a critical aspect of product development in the healthcare industry, involves identifying potential hazards associated with a device, estimating and evaluating the associated risks, controlling these risks, and monitoring the effectiveness of the controls put in place. This process is critical in ensuring the safety and effectiveness of medical devices. Medical device risk management is a comprehensive, lifecycle process that helps in making informed decisions about the safety of medical devices.
The process of medical device risk management is not a one-time event but an ongoing process that starts from the conception of a product and continues through its lifecycle. It is necessary to continually assess and re-assess the risks associated with a medical device. Factors such as new clinical information, technological advances, changes in regulatory requirements, and feedback from users may affect the risk profile of a device.
Medical device risk management, however, is not just about identifying and controlling risks. It also involves balancing risks against the benefits of a device. The ultimate goal is to ensure that the benefits of a medical device outweigh its risks, without compromising patient safety or device performance.
Importance of ISO 14971 in Medical Device Risk Management
ISO 14971, a key standard in the field of medical device risk management, provides a framework for managing risks related to medical devices. This standard is recognized globally and has become an essential part of the product development process in the healthcare industry. It provides a systematic approach to identifying hazards, estimating and evaluating the associated risks, controlling these risks, and monitoring the effectiveness of controls.
The implementation of ISO 14971 in medical device risk management ensures that a medical device meets the highest safety standards. It helps manufacturers to systematically uncover scenarios that could lead to injury or harm, enabling them to design safer products. Besides, it provides a structured approach to risk management that can be audited, which is crucial in demonstrating compliance with regulatory requirements.
ISO 14971 not only emphasizes the importance of a risk-based approach to the design and development of medical devices but also highlights the need for manufacturers to establish a risk management process. This process should be integrated into the organization’s quality management system and should be consistent with the overall policy and objectives of the organization.
Understanding the ISO 14971 Risk Management Process
The ISO 14971 risk management process is a comprehensive, systematic, and proactive approach to identifying, evaluating, controlling, and monitoring risks throughout the lifecycle of a medical device. The process involves several key steps, including
- risk analysis,
- risk evaluation,
- risk control, and
- production and post-production information.
Risk Analysis:
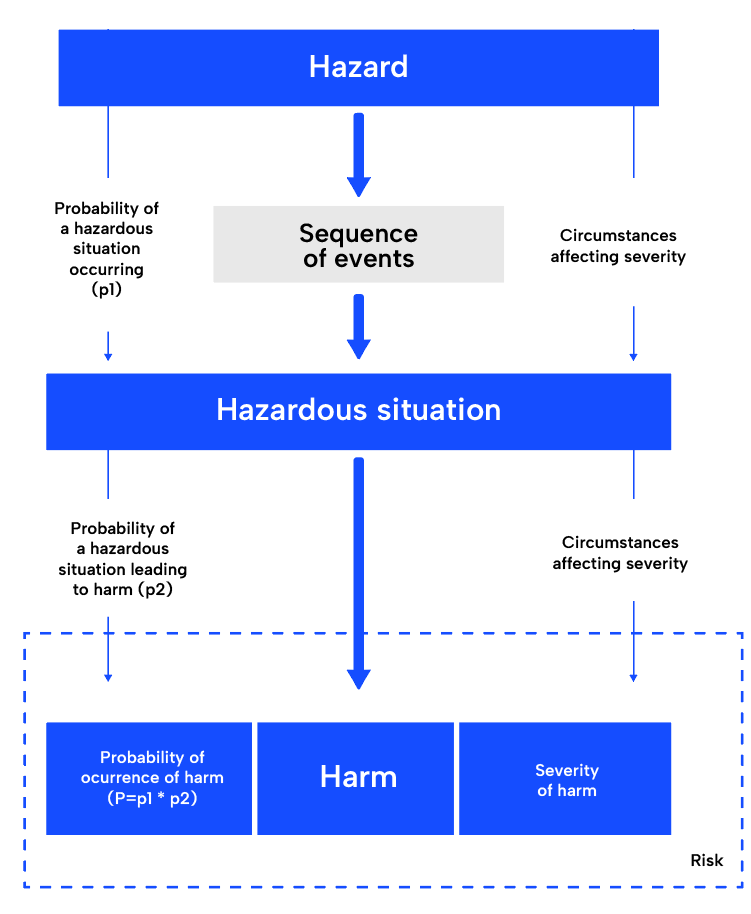
The first step in the ISO 14971 risk management process, risk analysis, involves understanding the intended use of the medical device and identifying potential hazards. It requires a thorough understanding of the device, its intended use, and the potential hazards that could arise during its use. Once identified, the potentially hazardous situations and the harms will be derived. A common tool is the failure mode & effects analysis (FMEA). It is very practical, as it already provides a structure following this approach.
Risk Evaluation:
The next step, risk evaluation, involves determining the severity and probability of each identified risk and deciding whether it is acceptable or needs further control measures.
Risk Control:
Risk control, the next step in the process, involves developing strategies to mitigate unacceptable risks or reduce even acceptable risks further. These strategies may include changes in the design of the device, providing protective measures in the medical device itself or the manufacturing process, and providing information to the users about the risks.
Overall residual risk evaluation
Once all risk mitigation strategies are in place and confirmed, the manufacturer is required to assess the total remaining risk associated with the medical device. This evaluation should consider all remaining risks and compare them to the benefits of the intended use. The methodology and the criteria for the acceptability of the aggregate residual risk should be as defined in the risk management plan. In cases where the aggregate residual risk is deemed acceptable, the manufacturer is obliged to notify users about any significant residual risks. Furthermore, they must provide the necessary information in the related documentation to communicate these remaining risks.
Post-market phase:
The final step in the process, production and post-production information, involves monitoring the medical device after it has been released to the market to ensure that it continues to meet safety requirements. This phase is crucial, as it will provide real-time data of the own device. A properly set up post-market surveillance process is important to provide solid data to feed in the risk management process.
Importance of Medical Device Hazard Analysis
Medical device hazard analysis, a significant component of the ISO 14971 risk management process, involves identifying and analyzing potential hazards associated with a medical device. This process is crucial in ensuring the safety and effectiveness of a device.
Hazard analysis helps in identifying potential sources of harm associated with a device. These could arise from the device itself, from the way it is used, or from the environment in which it is used. By identifying these hazards early in the design process, manufacturers can take steps to eliminate or mitigate them, thus preventing incidents that could lead to harm.
Hazard analysis also plays a crucial role in the risk evaluation process. By providing detailed information about potential hazards and their associated risks, it enables manufacturers to make informed decisions about the acceptability of these risks and the need for risk control measures. ISO 14971 provides in its annexes clear guidance to systematically identify potential hazards.
Implementing Risk Management 14971 in Product Design
Implementing risk management 14971 in product design ensures that medical devices are designed with safety in mind. The ISO 14971 standard provides a comprehensive framework for managing risks throughout the product design process, from the initial concept to the final product.
Implementing risk management 14971 in product design involves integrating the risk management process into the design and development process. This includes identifying potential hazards early in the design process, estimating and evaluating the associated risks, developing risk control measures, and monitoring the effectiveness of these measures.
By integrating risk management into the product design process, manufacturers can ensure that safety considerations are an integral part of the design process. This not only results in safer products but also reduces the likelihood of product recalls and liability claims.
Conclusion
In conclusion, the ISO 14971 standard plays a crucial role in medical device risk management. It provides a comprehensive framework for managing risks associated with medical devices, from the initial design to the final product. By encouraging a proactive, risk-based approach to product design, it helps manufacturers to design medical devices that are safe and effective.
Implementing the ISO 14971 standard in product design not only ensures the safety of medical devices but also contributes to their success. By identifying and controlling risks early in the design process, manufacturers can prevent incidents that could lead to harm, thus reducing the likelihood of product recalls and liability claims. Moreover, by evaluating the risks associated with a device against the benefits it provides, manufacturers can make informed decisions that contribute to the success of the product.
Talk to our experts today for more insights on implementing ISO 14971 in your medical device risk management process.




